Discovery using the isolated pathogen to develop a possible vaccine. Research is an extensive process that requires years to secure funding get approvals and.
 Sars Cov 2 Vaccines In Development Nature
Sars Cov 2 Vaccines In Development Nature
We invest in scientific and technical excellence to develop and launch a pipeline of new vaccines that meet the needs of patients and payers.

Vaccine development process. Generally in this phase vaccines are tested in young healthy adult volunteers. Vaccine research is costly. The current system for developing testing and regulating vaccines developed during the 20 th century as the groups involved standardized their procedures and regulations.
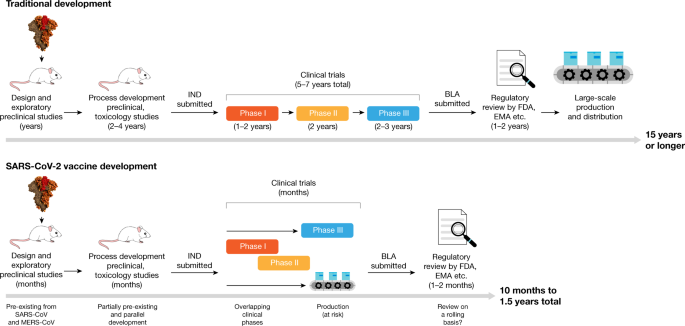
Viral vector-based vaccines constitute a promising part of the biopharmaceutical pipeline addressing many unmet indications. The typical vaccine development process This is the normal timeline for vaccine development. Figure 1Vaccine process development general approach Scientists have made significant breakthroughs in bioprocess and analytical technologies for supporting vaccine development.
The development cycle of a vaccine consists of six stages Exploratory stage Pre-clinical stage. Johns Hopkins International Vaccine Access Center Executive Director William Moss discusses ways to safely accelerate the coronavirus vaccine development process challenges in delivering a. The vaccine is given to a small number of volunteers to assess its safety confirm it generates an immune response and determine the right dosage.
Johnson Johnson Signs a Historic Pledge to Uphold the Integrity of the Scientific Process in Developing an Investigational COVID-19 Vaccine Every day that were able to accelerate the process could mean potentially hundreds even thousands of lives saved down the road says Macaya Douoguih MD MPH. A vaccine is a biological preparation that provides active acquired immunity to a particular infectious disease. In this step the infectious agent the virus or bacterium responsible for the disease is identified.
FDAs Center for Biologics Evaluation and Research CBER ensures that FDAs rigorous scientific and regulatory. After obtaining knowledge of the pathogen researchers can synthesize and have an mRNA vaccine ready for. Regulatory review and approval.
In 2018 a study in The Lancet Global Health estimated the cost of early development and initial clinical safety trials for a typical vaccine to be in the range of 31. Clinical development is a three-phase process. Scientists in these laboratories are most often funded by grants from the government or private foundations.
Typical vaccine development process starting in the lab through post-FDA-approval monitoring COVID-19 Vaccine Safety Surveillance Ongoing FDA monitoring of COVID-19 vaccine safety. If the vaccine triggers an immune response it is then tested in human clinical trials in three phases. Ensuring the safety and effectiveness of vaccines is one of FDAs top priorities.
The general stages of the development cycle of a vaccine are. Companies first make small batches and do small scale studies to characterise and optimise the production process. Standard vaccine development is a long process and studies are done in sequential steps.
Vaccine development is a long complex process often lasting 10-15 years and involving a combination of public and private involvement. Vaccine development typically begins not at a pharmaceutical company but in a research laboratory in a university medical center or small biotech company. Our work to research develop and introduce a new vaccine typically goes through several stages.
Understand the disease Recognizing and diagnosing the disease is the first step in the vaccine development process. Traditional vaccines are often grown in eggs or cells and then weakened or killed. A typical vaccine development timeline takes 5 to 10 years and sometimes longer to assess whether the vaccine is safe and efficacious in clinical trials complete the regulatory approval processes and manufacture sufficient quantity of vaccine doses for widespread distribution.
Such technologies have helped vaccine manufacturers achieve consistent product purity and quality rapidly and cost effectively. Several steps are required to make a vaccine. This is the period of time where scientists attempt to determine if they can identify a part of the virus which stimulates the immune memory so that the immune system can quickly destroy the pathogen before it can do harm.
To leverage the full potential of viral vector-based vaccine development many vaccine developers are looking to innovative technologies to support more productive intensified and flexible processes. They perform studies to determinate a suitable formulation that can keep vaccine components stable to the end of its shelf life. During Phase I small groups of people receive the trial vaccine.
A vaccine typically contains a biological preparation from disease-causing microorganism or since the beginning of the 21st century made synthetically that resembles it. Here is a summary of the steps.
Heres a look at their progress so far. DNA and RNA vaccines consist of Messenger RNA mRNA or DNA codefor making a version of a coronavirus protein.
 Hopes Rise For End Of Pandemic As Pfizer Says Vaccine Has 90 Efficacy World News The Guardian
Hopes Rise For End Of Pandemic As Pfizer Says Vaccine Has 90 Efficacy World News The Guardian
Many had completed the Phase III clinical trials a final step leading to.

Coronavirus vaccine development. In 2018 a study in The Lancet Global Health estimated the cost of early development and initial clinical safety trials for a. Since early 2020 vaccine development has been expedited via unprecedented collaboration in the multinational pharmaceutical industry and between governments. After a coronavirus was isolated in December 2019 its genetic sequence was published on 11 January 2020 triggering an urgent international response to prepare for an outbreak and hasten development of a preventive COVID-19 vaccine.
Prior to the COVID19 pandemic work to develop a vaccine against coronavirus diseases like severe acute respiratory syndrome SARS and Middle East respiratory syndrome MERS established. Companies first make small batches and do small scale studies to characterise and optimise the production process. There are more than 50 clinical trials worldwide.
The COVID-19 vaccine like other vaccines works by training our bodies to develop antibodies to fight against the virus that causes COVID-19 to prevent future illness. Peter Marks director of FDAs Center for Biologics Evaluation and Research to discuss the basics of COVID-19 vaccine development. That vaccine could then be customized to fight different coronaviruses.
More information regarding Australias vaccine program is available at the Department of Health webpage. Researchers around the world are developing vaccines against SARS-CoV-2 the virus that causes COVID-19. The code is inserted into a human cell which then uses the genetic instructions to.
WHO and its partners are committed to accelerating the development of COVID-19 vaccines while maintaining the highest standards on safety. As of December 28 2020 large-scale Phase 3 clinical trials are in progress or being planned for three COVID-19 vaccines in the United States. Both are currently being administered in the US.
The RD Blueprint was activated to accelerate diagnostics vaccines and therapeutics for this novel coronavirus. The COVID-19 pandemic caused by the SARS-CoV-2 coronavirus has afflicted millions of people worldwideThis global crisis has spurred dozens of vaccine development efforts by drug companies universities and governments. In the past vaccines have been developed through a series of steps that can take many years.
A typical vaccine development timeline takes 5 to 10 years and sometimes longer to assess whether the vaccine is safe and efficacious in clinical trials complete the regulatory approval processes and manufacture sufficient quantity of vaccine doses for widespread distribution. Finally based on these prior vaccination experiences we discuss recent progress and potential challenges of COVID-19 vaccine development. The TGA is actively monitoring COVID-19 vaccine development in Australia and around the world and is part of a network of international regulators that meet regularly to discuss the development of COVID-19 vaccines.
Finally cutting-edge vaccines under development rely on deploying pieces of the coronaviruss genetic material enabling our cells to temporarily make coronavirus proteins needed to stimulate. Around 150 vaccines were in various stages of development across the globe as of mid December 2020. Standard vaccine development is a long process and studies are done in sequential steps.
A COVID19 vaccine is a vaccine intended to provide acquired immunity against severe acute respiratory syndrome coronavirus 2 SARSCoV2 the virus causing coronavirus disease 2019 COVID19. The Blueprint aims to improve coordination between scientists and global health professionals accelerate the research and development process and develop new norms and standards to learn from and improve upon the global response. They perform studies to determinate a suitable formulation that can keep vaccine components stable to the end of its shelf life.
There is currently no evidence that antibodies formed from COVID-19 vaccination cause any problems with pregnancy including the development of the placenta. Severe Acute Respiratory Syndrome Coronavirus 2 SARS-CoV-2 is a new type of coronavirus that causes the Coronavirus Disease 2019 COVID-19 which has been the most challenging pandemic in this century. In Part 1 of FDA Insights vaccine series Dr.
Funding for COVID-19 vaccine research Vaccine research is costly. The Moderna COVID-19 vaccine has been authorized for emergency use by the FDA under an Emergency Use Authorization EUA on December 18 2020. Vaccines typically require years of research and testing before reaching the clinic but in 2020 scientists embarked on a race to produce safe and effective coronavirus vaccines in record time.
COVID-19 vaccine development has been lightning-fast Several acceleration efforts are currently underway like the White Houses Operation Warp Speed which is meant to cut through regulatory red. The scientists chose to focus on one prototype coronavirus and create a vaccine for it. Years before the COVID-19 pandemic began experts at the NIH Vaccine Research Center VRC were studying coronaviruses to find out how to protect against them.
Search This Blog
Labels
- 1000
- 10000
- 1000an
- 1040
- 1080p
- 1080x2160
- 10th
- 1366x768
- 13th
- 1920x1080
- 1969
- 19th
- 2003
- 2006
- 2012
- 2013
- 2015
- 2016
- 2017
- 2018
- 2019
- 2020
- 2021
- 2022
- 2048
- 24th
- 2560x1440
- 2k20
- 30th
- 45th
- 50th
- abbott
- abbreviation
- about
- above
- abstract
- acanthus
- acar
- accept
- accord
- account
- accounting
- aceh
- acres
- activate
- activities
- actor
- actress
- adalah
- addition
- address
- adhesive
- adidas
- aditya
- adults
- advil
- aesthetic
- agency
- ages
- aggarwal
- agreement
- aiko
- aladdin
- alberta
- album
- alfredo
- algebra
- alien
- alienware
- allergic
- alphabet
- alpukat
- amazing
- amazon
- american
- among
- anatomy
- android
- aneka
- animal
- animals
- animated
- anime
- ankle
- anne
- anniversary
- annuity
- anom
- answers
- anti
- antique
- apart
- apartment
- apartments
- appearance
- apple
- applied
- apply
- approved
- aquarium
- arabic
- arcade
- arceus
- area
- arguments
- arizona
- army
- around
- arrays
- articuno
- arts
- asam
- asem
- asian
- asin
- asli
- asparagus
- assistant
- asta
- astra
- atlanta
- attach
- audubon
- aurora
- australia
- australias
- auto
- automobile
- autumn
- avengers
- awesome
- awuk
- ayam
- babat
- babi
- babies
- baby
- back
- backdrops
- background
- backgrounds
- backs
- backyard
- badges
- badlands
- badu
- bahan
- bailey
- bajaj
- bakar
- bakery
- bakpao
- bakso
- balaji
- balakutak
- balance
- bali
- ball
- balls
- balok
- band
- bandung
- bangs
- bape
- bark
- baroque
- basah
- base
- basic
- basket
- basketball
- batagor
- bathroom
- bayam
- bayi
- beach
- bean
- bear
- beard
- bears
- beat
- beatrice
- beautiful
- bebek
- beckett
- become
- bedroom
- beginner
- beginners
- beige
- bein
- bekas
- belanda
- bellwork
- belt
- benefits
- bening
- bermuda
- best
- betawi
- between
- bhagavad
- bhagwan
- bible
- biggest
- bike
- bikin
- bikinis
- bill
- binod
- birch
- birds
- birthday
- black
- blackmilk
- blank
- Blank Last Will Form Free Florida
- blanket
- bless
- blick
- blossom
- bluder
- blue
- blur
- blush
- board
- bobber
- boho
- bolen
- bollywood
- bolu
- bombay
- book
- boost
- booster
- border
- borders
- boston
- botanical
- botok
- bottle
- bouquet
- boxing
- boys
- brady
- brain
- brand
- brands
- brazilliance
- bread
- breaker
- breast
- breeds
- brick
- bridge
- bright
- britto
- broker
- bronco
- bronx
- brooklyn
- brother
- brownies
- brush
- brushes
- bryant
- bryce
- buah
- buat
- bubur
- buddha
- budget
- buffalo
- build
- bumbu
- buncis
- burger
- business
- butter
- butterfly
- butterscotch
- buttons
- buying
- buzzfeed
- bypass
- cabe
- cabinet
- cable
- cache
- cactus
- cafe
- cake
- cakes
- calculate
- calculator
- calendar
- calico
- california
- call
- calla
- calligraphy
- calories
- camaro
- camera
- cameras
- camo
- campervan
- canada
- cancer
- candil
- canson
- canva
- canvas
- capitalization
- cara
- carbonara
- card
- cardi
- cards
- care
- carp
- carpenter
- cars
- cartoon
- case
- cases
- casino
- casio
- cassidy
- cast
- castle
- catcher
- cats
- cave
- cbr1000rr
- ceiling
- ceker
- cell
- cemilan
- cempedak
- center
- ceramic
- ceremony
- ceria
- certificate
- chair
- chairs
- challenger
- championship
- change
- channel
- charcoal
- charger
- charizard
- charms
- chart
- chase
- cheap
- cheats
- check
- chelsea
- cherry
- chicago
- chicken
- chickenpox
- chiefs
- child
- chinese
- chip
- chocolatos
- cholesterol
- chop
- chore
- christian
- christmas
- chrome
- churros
- cilantro
- cilok
- cilor
- cincang
- cincau
- cinderella
- circulation
- cirebon
- city
- civic
- clans
- class
- classes
- clean
- clear
- clip
- clipart
- clippers
- clips
- clock
- clothes
- cloud
- clouds
- cloze
- cmyk
- coca
- code
- coffee
- coffin
- coklat
- cola
- cold
- collage
- collection
- colo
- color
- colorado
- colorful
- coloring
- colour
- colourful
- colours
- column
- combine
- combined
- combo
- comfort
- commentaries
- commercial
- companies
- company
- comparison
- compensation
- complaints
- comprehension
- computer
- concept
- concrete
- confias
- connect
- conquest
- construction
- consulting
- container
- content
- context
- controller
- convert
- cook
- cookies
- cool
- coordinate
- coordinates
- copyright
- coral
- cord
- corinthians
- coritiba
- cork
- cornflakes
- coronavirus
- cost
- costco
- costumes
- cottage
- cough
- country
- countryside
- couple
- coupon
- coupons
- courthouse
- cover
- coverings
- covid
- cowboy
- cowpox
- craft
- craigslist
- crashers
- cream
- creamer
- create
- creative
- creator
- crepes
- cricut
- crispy
- cristiano
- croc
- cross
- crossword
- crosswords
- crying
- cuba
- cube
- cubit
- cuciwis
- cucur
- cumi
- cups
- cursive
- custom
- customize
- cute
- cyanide
- cynthia
- dadar
- dads
- daging
- daily
- daisy
- dalgona
- dance
- dangerous
- dangers
- dapp
- daredevil
- dari
- dark
- dasar
- dashboard
- date
- daughter
- davidson
- davis
- daycare
- deadpool
- death
- debby
- debit
- decimal
- decimals
- decks
- deco
- decor
- decoration
- decorations
- decorative
- definition
- delhi
- deli
- delisted
- delivery
- dello
- dementia
- demon
- denali
- dencis
- dendritic
- dentist
- desert
- design
- designer
- designs
- desktop
- dessert
- development
- devi
- device
- diabetes
- diana
- diet
- difference
- different
- digi
- digit
- digital
- dijual
- dinosaur
- disable
- discord
- discount
- discovery
- disease
- disembunyikan
- dishwasher
- disney
- distributed
- distribution
- doctorate
- doctors
- dodge
- does
- dogs
- doll
- donat
- donghia
- donkey
- donut
- doodle
- doorbell
- dora
- dose
- doses
- doughnuts
- download
- downloads
- dragon
- dragonite
- Drama
- draw
- drawer
- drawing
- dress
- dresses
- driftwood
- dualshock
- duck
- ducks
- dunkin
- durga
- during
- dutch
- duty
- eagle
- earbuds
- earth
- easy
- echo
- eclectic
- edge
- editor
- eevee
- eeveelution
- effect
- effective
- effectiveness
- effects
- efficacy
- ekonomis
- elapsed
- electric
- elegant
- elements
- elephant
- elite
- elves
- emirates
- emoji
- emotional
- empal
- empek
- employer
- empuk
- emulator
- enak
- enchanted
- endangered
- ending
- energy
- engagement
- engine
- england
- english
- enoki
- enough
- enrichment
- episodes
- ereading
- erie
- erykah
- escape
- esport
- esports
- espresso
- etsy
- eucalyptus
- euthanize
- evening
- evil
- evolve
- exact
- example
- excel
- excuse
- exercises
- expanded
- expansion
- expat
- experiments
- explorer
- eyes
- fabric
- face
- facetime
- facts
- facultad
- fairy
- fall
- Family
- famous
- farm
- feather
- feeding
- feet
- female
- fence
- fencing
- fern
- fettuccine
- fever
- fifth
- figure
- file
- filling
- filters
- financial
- find
- finder
- fire
- fireblade
- firefighter
- fires
- first
- fish
- fishing
- fist
- fitness
- fixtures
- flag
- flat
- flatten
- flavors
- fletcher
- flight
- flip
- floor
- flooring
- floral
- florida
- Florida Will Forms Free Printable
- Florida Wills Free Forms Blank
- flour
- flower
- flowers
- flucelvax
- fluffy
- fluid
- foggy
- followers
- fondant
- font
- food
- foot
- footage
- football
- footnote
- ford
- forest
- fork
- form
- formal
- format
- forms
- fort
- fortnite
- foto
- found
- foundation
- four
- fractions
- frame
- framed
- francisco
- free
- Free Florida Will and Testament Printable Form
- Free Florida Will Codicil Form
- freepik
- french
- frenzy
- friday
- fried
- friendly
- friends
- frock
- from
- front
- frozen
- fryer
- fudgy
- full
- function
- funny
- furniture
- futuristic
- fvrcp
- gagal
- galantin
- galaxy
- gallery
- gambar
- game
- games
- gaming
- ganesh
- ganpati
- garden
- gardens
- garena
- gather
- gator
- gautam
- gautama
- geico
- gelas
- genealogy
- general
- generator
- geneva
- gentong
- georgia
- geprek
- ghibli
- giant
- gift
- gifts
- gila
- giovanna
- giraffe
- girl
- girlfriend
- girls
- gita
- given
- glaceon
- glass
- glasses
- glitch
- glitter
- global
- globe
- glucose
- gluten
- goal
- godog
- gogeta
- gogh
- goku
- gold
- golden
- goldendoodle
- goldilocks
- golf
- good
- goreng
- gorgeous
- gori
- gown
- grade
- graders
- graffiti
- grammar
- graphic
- grass
- gratis
- gray
- great
- greek
- green
- greenhouse
- gregg
- grey
- grid
- grieving
- grill
- grocery
- gronk
- groom
- growlithe
- guardianship
- gucci
- guide
- guitar
- gulab
- gulai
- gulung
- guppies
- gurih
- hack
- hacked
- hair
- haircut
- halen
- half
- halfpipe
- halloween
- hand
- handicap
- handwriting
- hanging
- hanukkah
- hanuman
- happy
- harassment
- harga
- hari
- harley
- harper
- harvest
- hatcher
- have
- headphones
- health
- heart
- heartgold
- heels
- hell
- hello
- help
- hepatitis
- herb
- herd
- heroine
- heroines
- herringbone
- hidden
- hide
- high
- highest
- highlights
- hijau
- hill
- hillside
- hindi
- historic
- hitam
- hobby
- holder
- holiday
- hollywood
- home
- homemade
- homeowners
- homework
- honda
- honeywell
- horizons
- horizontal
- Horror
- horse
- horses
- horseshoes
- hospital
- house
- howard
- hubble
- huge
- humans
- hunting
- husband
- hyacinth
- iced
- icon
- idea
- ideas
- ikan
- ikea
- illusion
- image
- images
- imdb
- immune
- imovie
- imposter
- improve
- incense
- income
- increase
- india
- indian
- indigo
- indonesia
- inexpensive
- infant
- infinity
- influence
- influenza
- informational
- ingkung
- initial
- injury
- insert
- inspiration
- inspired
- instacart
- instant
- instinct
- insurance
- intact
- interactive
- interesting
- interfacelift
- interruption
- into
- invented
- invitation
- ipad
- iphone
- ireland
- islamic
- island
- itachi
- italian
- jack
- jackalope
- jadah
- jadul
- jagung
- jajanan
- jakarta
- jameson
- jamur
- japan
- japanese
- jawa
- jeep
- jennings
- jents
- jerry
- jesus
- joann
- jobs
- johnson
- johto
- journal
- jualan
- july
- jungkook
- jungle
- jurassic
- kaaba
- kabsah
- kacang
- kaiser
- kajal
- kaki
- kaktus
- kalasan
- kambing
- kandangan
- kangkung
- kaos
- kapoor
- karakter
- karamel
- kareem
- kari
- kartika
- kawaii
- kecambah
- kecap
- keep
- keju
- kekinian
- kelapa
- kelinci
- kemangi
- kembung
- kemiri
- kemplang
- kenikir
- kennel
- kentang
- kenworth
- kepala
- kepiting
- keren
- kering
- kerupuk
- ketan
- ketawa
- kettle
- ketupat
- keyboard
- khas
- kids
- kimchi
- kimlo
- kindergarten
- kinetic
- king
- kitchen
- kittens
- kitty
- klepon
- know
- kobe
- kocok
- kokoh
- kolkata
- konidela
- kontinental
- kopi
- kotel
- krecek
- kris
- krishan
- krishna
- krispi
- kuah
- kudus
- kukus
- kumon
- kuning
- kuwut
- kyojin
- label
- labels
- labradors
- labu
- ladies
- lakshmi
- lamongan
- landscape
- landscaping
- lanka
- lantern
- lapis
- laptop
- large
- largest
- last
- latest
- latin
- laut
- lavazza
- lawn
- lawyer
- laxmi
- lazy
- leaf
- league
- learning
- leather
- leaves
- lebaran
- lebowski
- left
- legal
- legit
- lembut
- lemon
- lemongrass
- lemper
- lesson
- lessons
- letter
- letters
- level
- lewis
- lexus
- liability
- license
- lidi
- lids
- life
- lifestyle
- light
- lightning
- like
- lilac
- lily
- limo
- limoncello
- line
- linen
- lion
- lippo
- liquid
- list
- lite
- live
- living
- llama
- lobby
- location
- locations
- lock
- logic
- logo
- london
- long
- longboard
- look
- lord
- lotr
- lounge
- love
- lovers
- luar
- lumut
- lurantis
- lure
- luther
- lyme
- lyrics
- macaroni
- macbook
- machine
- machu
- madison
- magnet
- magnetic
- magnolia
- mahalaxmi
- main
- mainboard
- maintenance
- maizena
- makanan
- makassar
- make
- maker
- making
- maklor
- malaria
- male
- mamba
- mana
- manager
- mandatory
- manger
- manis
- mantoux
- marble
- march
- marimekko
- market
- marketplace
- maroon
- marriage
- martabak
- martial
- martin
- marvel
- marzocco
- masak
- masakan
- mashed
- mask
- mason
- mata
- matang
- match
- math
- maths
- matrix
- mature
- mayor
- maze
- mazes
- mcalisters
- mcdonalds
- meaning
- measles
- measure
- medical
- medicare
- medicina
- mehandi
- melhores
- membrane
- membuat
- meme
- memes
- menampilkan
- menards
- meningitis
- meningococcal
- mentai
- mento
- menu
- merah
- mercedes
- meriah
- merry
- messages
- metal
- metallic
- meth
- mexican
- mica
- michaels
- michelangelo
- microsoft
- midnight
- mini
- minimalist
- minneapolis
- mint
- minute
- mirror
- missing
- mobile
- mockup
- model
- modern
- moderna
- mods
- molen
- momentos
- money
- monopoly
- moon
- morgan
- moroccan
- mosaic
- moss
- mother
- motivational
- motogp
- motor
- motorcycle
- mount
- mountain
- mounted
- moveable
- moveset
- movie
- movies
- moving
- movistar
- mpasi
- mpek2
- mrna
- much
- muda
- mudah
- muffin
- mujair
- multi
- multiplication
- multiply
- mummy
- mural
- murals
- muriels
- muscle
- Music
- mustang
- mutiara
- Mystery
- mystic
- nail
- name
- names
- nanas
- napoli
- narayan
- narrow
- nashville
- nasi
- nastar
- natal
- national
- native
- nativity
- natural
- nature
- navy
- near
- nearest
- need
- negeri
- neon
- nescafe
- nespresso
- newborn
- News
- nextwall
- nice
- night
- niharika
- nike
- nila
- ninja
- nintendo
- nissan
- nocturnal
- note
- notebook
- notecards
- nouns
- novavax
- november
- nugget
- number
- numbers
- numerals
- nurse
- nursery
- nutrijell
- nuzlocke
- nwea
- nyat
- nytimes
- oakland
- oatmeal
- obat
- object
- oblok
- ocean
- oenis
- office
- official
- officiant
- often
- ohio
- oklahoma
- olahan
- older
- oldies
- olds
- oles
- olos
- olympics
- oncom
- online
- only
- ontario
- oobleck
- open
- opposites
- orange
- ordained
- organization
- origami
- original
- othello
- outbreak
- outdoor
- outlet
- outlook
- oven
- oversized
- pack
- packs
- padang
- padeh
- page
- pages
- paid
- painlessly
- paint
- paintable
- painted
- painting
- paintings
- pajamas
- pakistan
- palace
- paleo
- pallet
- palm
- pancake
- panchmukhi
- panda
- panel
- panggang
- panjang
- panther
- paper
- park
- parlay
- parsley
- partnership
- party
- parvati
- pass
- passages
- pasta
- pastel
- pathologist
- pathology
- patrick
- patriotic
- patriots
- patrol
- pattern
- patterns
- pattinson
- payment
- peach
- peacock
- peaky
- pearson
- pecak
- pedagang
- peel
- pempek
- pencil
- pendant
- penguin
- penius
- pens
- people
- pepes
- perhitungan
- perisai
- perkedel
- persian
- persona
- personalized
- pets
- pexels
- pfizer
- pharmacy
- philadelphia
- philippines
- phone
- phones
- phonics
- photo
- photography
- photos
- photoshoot
- pick
- pics
- picture
- pictures
- piece
- pieces
- piercings
- pigeon
- pineapple
- pink
- pisang
- pitbull
- pizza
- place
- plaid
- plan
- planner
- plans
- plant
- plants
- platelets
- plates
- platinum
- play
- playing
- playstation
- plug
- plus
- plush
- pneumococcal
- pneumonia
- pokedex
- pokemon
- poker
- police
- polio
- poll
- polos
- polska
- popular
- populer
- pork
- portrait
- positive
- possessive
- poster
- postponed
- potato
- potatoes
- potted
- powder
- power
- practice
- practicing
- praktis
- pray
- pregnancy
- pregnant
- premier
- premium
- preschool
- preschoolers
- preservative
- press
- prettiest
- pretty
- prevent
- price
- primary
- prime
- princess
- printable
- printables
- prints
- priority
- private
- problem
- problems
- process
- products
- professional
- project
- promo
- pronunciation
- pros
- puasa
- puding
- pukis
- pulsar
- puma
- pumpkin
- puppets
- puppies
- puppy
- purple
- pusheen
- putih
- putu
- qatar
- quadrivalent
- quality
- quantity
- queen
- querytablesadd
- quest
- quiz
- quote
- quotes
- rabies
- racing
- radha
- radhe
- radio
- rainbow
- rainforest
- raise
- rajasthan
- rambutan
- rancevi
- randy
- rates
- ratio
- rayner
- reaction
- read
- readiness
- reading
- real
- really
- rebel
- reception
- recife
- recipe
- rectangular
- redding
- refrigerator
- regal
- registry
- regrouping
- release
- released
- removable
- remove
- rendang
- rent
- renters
- renyah
- replace
- report
- repsol
- resep
- resolution
- resources
- restaurant
- retro
- review
- reviews
- rfid
- rhode
- rica
- rifle
- rigged
- rims
- ring
- rings
- ringtones
- ritter
- road
- robert
- robot
- rock
- rocky
- roll
- romanized
- ronald
- room
- roosevelt
- rose
- ross
- roti
- round
- royalty
- rubella
- rujak
- rules
- rumahan
- running
- rupees
- russian
- rustic
- ryan
- safe
- sage
- saikoro
- sajiku
- sakura
- salad
- sale
- salle
- salmon
- salon
- saltwater
- sambal
- sambel
- samsung
- santa
- santan
- saori
- sapi
- saraswati
- saree
- sari
- sars
- sate
- sauce
- saucer
- sawi
- sayings
- sayur
- scarf
- scene
- scenery
- scenes
- schedule
- schneider
- school
- science
- scientific
- score
- scottsdale
- scouting
- scrabble
- scramble
- scrapbook
- screen
- screensavers
- screenshot
- script
- sculpture
- seagrass
- sealed
- search
- seascape
- seasonal
- seasons
- seattle
- seblak
- second
- secret
- secrets
- sederhana
- sehari
- selatan
- self
- sell
- semprit
- senayan
- send
- sending
- senior
- seniors
- sensory
- sentences
- sera
- serba
- serealia
- serebii
- serut
- server
- service
- services
- setting
- seven
- shabby
- shadow
- shamrock
- shape
- shapes
- share
- shareable
- sheet
- sheets
- shells
- shelter
- shield
- shingles
- shingrix
- shiplap
- shirt
- shirts
- shiv
- shoes
- shoot
- shop
- Short
- shortest
- should
- shower
- shows
- shri
- shutter
- shutterstock
- side
- siege
- sight
- sign
- signature
- signs
- silhouette
- silver
- simple
- singer
- singkong
- single
- siomay
- sirius
- sirloin
- sister
- site
- sites
- sixth
- size
- sizes
- sketchbook
- skunk
- skyline
- skyrim
- slayer
- slim
- slope
- slot
- small
- smallpox
- smart
- smartphone
- smartwatch
- smoothies
- snail
- snails
- snapchat
- snowfall
- snowflake
- sobro
- social
- soda
- sofascore
- soil
- solid
- solutions
- song
- songs
- sony
- sosis
- soto
- soul
- sound
- sounds
- south
- space
- sparkle
- spelling
- spesial
- spiderman
- splash
- splatter
- splenectomy
- spoofer
- spoon
- Sport
- sports
- sporty
- spotify
- spring
- sprite
- srikaya
- stadium
- stages
- stainless
- stand
- standard
- star
- starbucks
- starfleet
- starters
- state
- states
- stats
- status
- steam
- steel
- steelers
- step
- stevie
- stick
- stickers
- stiletto
- stitch
- stock
- stocks
- stop
- storage
- store
- stories
- stormtrooper
- story
- strain
- stranger
- strap
- strategy
- streaming
- street
- stripe
- striped
- structural
- stubs
- stup
- style
- styles
- subaru
- subtraction
- sugarboo
- suki
- sultana
- sumsum
- sunflower
- sunny
- sunset
- sunshine
- super
- superkids
- supermarket
- supply
- support
- supra
- supreme
- surface
- surreal
- susu
- sutra
- swami
- swan
- swimming
- swine
- switch
- sword
- symbol
- sympathy
- syracuse
- system
- table
- tablet
- taekwondo
- tahu
- taichan
- takaran
- take
- taks
- talking
- tamil
- tank
- tanpa
- tape
- target
- tarkov
- tart
- tartan
- tattoo
- tattoos
- tauge
- tawar
- tcgplayer
- tdap
- teach
- teachers
- team
- teams
- teddy
- teenage
- teeth
- tegal
- telephone
- television
- televisions
- telur
- tempaper
- tempe
- temperature
- template
- templates
- temporary
- tengah
- tenggiri
- tengkleng
- tenses
- tepung
- teri
- terigu
- teriyaki
- terlambat
- terrier
- test
- testosterone
- tetanus
- texas
- text
- textured
- thai
- thank
- thanksgiving
- that
- theft
- their
- theme
- there
- thermostat
- things
- third
- this
- thousandths
- throw
- thumbs
- tiger
- tigger
- tiktok
- tile
- tiles
- time
- timed
- timeline
- times
- tinder
- tins
- tintin
- title
- toast
- todd
- toddler
- together
- toilet
- tokyo
- toned
- tongkol
- tongseng
- tool
- toppers
- toronto
- totodile
- tournament
- towers
- toyota
- trace
- tracing
- tracker
- tracking
- tractor
- train
- trainer
- training
- transfer
- transfers
- transitions
- transparent
- tray
- tree
- trees
- trek
- trial
- triangle
- trip
- trippie
- trippy
- trout
- truck
- tuesday
- tulang
- tulip
- tumblers
- tumblr
- tumis
- tumisan
- tumit
- tune
- turkey
- turkeys
- turn
- turntable
- tutorial
- tutorials
- twin
- type
- types
- uanl
- ucapan
- uchiha
- udang
- ultra
- ultras
- umami
- uncharted
- unconstitutional
- under
- understanding
- unemployment
- ungu
- unicorn
- unik
- uninstall
- unique
- unit
- united
- units
- unlimited
- unlock
- unsplash
- untuk
- update
- upholster
- urban
- urchin
- urine
- used
- username
- using
- utamakan
- vaccinate
- vaccinating
- vaccination
- vaccine
- vaccines
- valentine
- valhalla
- value
- valve
- varicella
- vector
- vegas
- vegeta
- vegetable
- vegetarian
- vehicle
- venkatajalapathi
- venkateswara
- venue
- verified
- versace
- verse
- verses
- very
- veterans
- video
- videos
- vietnam
- villagers
- vintage
- vinyl
- vocabulary
- voice
- volkswagen
- vomiting
- vulpera
- wala
- wall
- wallet
- wallpaper
- wallpapers
- wallpops
- walls
- walmart
- walpeper
- wangi
- warfare
- warm
- wars
- wash
- watch
- watches
- water
- watercolor
- watercolors
- waterfall
- waterhouse
- wave
- wayfair
- waylon
- wear
- website
- websites
- wedding
- welcome
- went
- west
- what
- whats
- when
- where
- which
- while
- white
- wholesale
- widescreen
- wikipedia
- wild
- wildlife
- will
- window
- windows
- windshield
- wine
- wingko
- winter
- wireless
- wishes
- wisman
- with
- without
- wolf
- wolverines
- woman
- wonder
- wood
- wooden
- wooloo
- word
- words
- work
- workbook
- works
- worksheet
- worksheet1
- worksheets
- world
- wortel
- worth
- wrangler
- wrap
- write
- writing
- xmas
- yahtzee
- yang
- yantra
- yard
- year
- years
- yeezy
- yellow
- york
- young
- your
- youtube
- zebra
- zedge
- zenica
- zeppelin
- zero
- zombies
- zoomquilt
- zusammenführen
